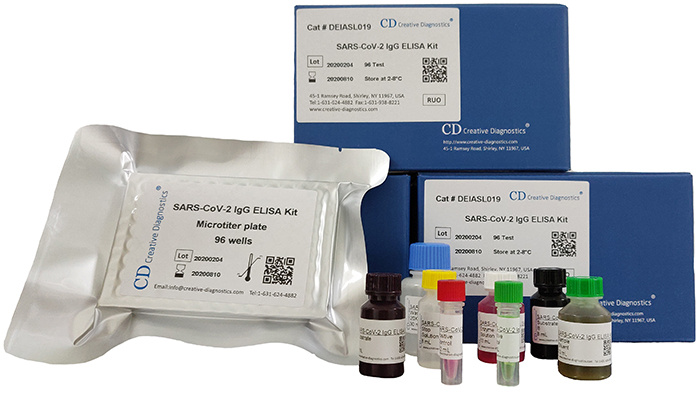
SARS-CoV-2 Immunoassay Kits, 96T
Kód produktu: DEIASL020
Kód výrobce: DEIASL020
Výrobce:
Creative Diagnostics, A Division of CD Biosciences, Inc.
Detailní popis
Size
96T
Sample
Serum, plasma
Intended Use
This kit is used for the qualitative detection of novel coronavirus IgM antibodies in human serum or plasma in vitro.
Storage
This kit is stored at 2 ~ 8 °C, the validity period is 6 months.
Avoid freezing and use within the validity period.
Production date, valid until the label.
Avoid freezing and use within the validity period.
Production date, valid until the label.
General Description
The new coronavirus belongs to the beta coronavirus of the genus β, which has an envelope, the particles are round or oval, often polymorphic, and the diameter is 60-140nm. Its genetic characteristics are significantly different from SARSr-CoV and MERSr-CoV. Current research shows that it has more than 85% homology with bat SARS-like coronavirus (bat-SLCoVZC45).
In vitro isolation and culture, 2019-nCoV can be found in human respiratory epithelial cells in about 96 hours, while it takes about 6 days to isolate and culture in Vero E6 and Huh-7 cell lines. Based on current epidemiological investigations, the incubation period is generally 7 days, with a maximum of 14 days. Main symptoms are fever, fatigue, and dry cough. A few patients have symptoms such as nasal congestion, runny nose, and diarrhea. In severe cases, dyspnea occurs more than a week later. In severe cases, acute respiratory distress syndrome, septic shock, difficult to correct metabolic acidosis, and coagulation dysfunction develop rapidly. It is worth noting that in the course of severe and critically ill patients, there may be moderate to low fever, even without obvious fever. Some patients showed only low fever, mild fatigue, and no pneumonia and recovered after 1 week. In the early stages of the disease, the total number of white blood cells in the peripheral blood was normal or decreased, the lymphocyte count decreased, and some patients had increased liver enzymes, muscle enzymes, and myoglobin. Most patients have elevated C-reactive protein (CRP) and erythrocyte sedimentation rate and normal procalcitonin. In severe cases, D-dimer increases and peripheral blood lymphocytes progressively decrease. New coronavirus nucleic acids can be detected in throat swabs, sputum, lower respiratory tract secretions, and blood. [1] Serum antibody testing helps confirm the infection status of a case.
In vitro isolation and culture, 2019-nCoV can be found in human respiratory epithelial cells in about 96 hours, while it takes about 6 days to isolate and culture in Vero E6 and Huh-7 cell lines. Based on current epidemiological investigations, the incubation period is generally 7 days, with a maximum of 14 days. Main symptoms are fever, fatigue, and dry cough. A few patients have symptoms such as nasal congestion, runny nose, and diarrhea. In severe cases, dyspnea occurs more than a week later. In severe cases, acute respiratory distress syndrome, septic shock, difficult to correct metabolic acidosis, and coagulation dysfunction develop rapidly. It is worth noting that in the course of severe and critically ill patients, there may be moderate to low fever, even without obvious fever. Some patients showed only low fever, mild fatigue, and no pneumonia and recovered after 1 week. In the early stages of the disease, the total number of white blood cells in the peripheral blood was normal or decreased, the lymphocyte count decreased, and some patients had increased liver enzymes, muscle enzymes, and myoglobin. Most patients have elevated C-reactive protein (CRP) and erythrocyte sedimentation rate and normal procalcitonin. In severe cases, D-dimer increases and peripheral blood lymphocytes progressively decrease. New coronavirus nucleic acids can be detected in throat swabs, sputum, lower respiratory tract secretions, and blood. [1] Serum antibody testing helps confirm the infection status of a case.
Hodnocení produktu
Produkt zatím nikdo nehodnotil, buďte první!